As of February 7, 2014 at 4:00 p.m. Eastern Time, shares of Intercept were trading in the range of $301.03 to $352.80. The average trading volume was 1.22 million shares. Currently, there are 19.34 million shares outstanding and the insiders own approximately 42% of the shares outstanding. Intercept has 53% institutional ownership interest. The market cap for the company is $6.80 billion. During the past 52 weeks, shares of the company traded from $30 to $497.
(click to enlarge)
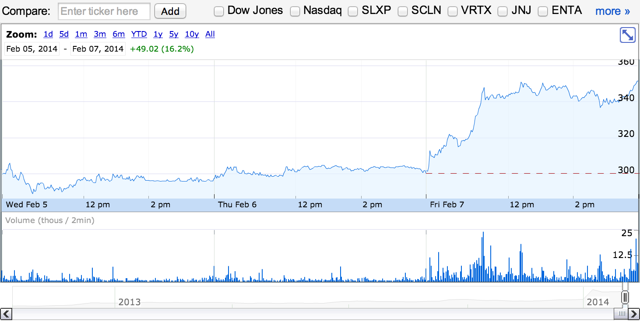 Source: Google Finance
Source: Google FinanceEarlier this week, Intercept issued a press release stating that Doctor Pruzanski, President and CEO, is going to present at the Leerink Global Healthcare Conference in New York on Thursday, February 13, 2014. I recommend investors to attend this event to learn more information regarding Intercept and the company's exciting products-in-development.
NEW YORK, Feb. 5, 2014 (GLOBE NEWSWIRE) -- Intercept Pharmaceuticals, Inc., a clinical stage biopharmaceutical company focused on the development and commercialization of novel bile acid therapeutics to treat chronic liver and intestinal diseases, today announced that Mark Pruzanski, M.D., President and Chief Executive Officer, will present at the Leerink Global Healthcare Conference in New York on Thursday, February 13, 2014 at 2:15 p.m. Eastern Time.Intercept's lead product (OCA) is a semi-synthetic bile acid that has an ethyl group added to the 6-C position on the molecule. This ingenious chemical modification conferred OCA with 100x potency in activating FXR versus natural bile acid (CDCA). Based on my research, I believe that FXR is the "holy grail receptor" … for the activation of FXR elicits a plethora of key physiologic responses.
Intercept is a biopharmaceutical company focused on the development and commercialization of novel therapeutics to treat orphan and more prevalent liver and intestinal diseases utilizing its expertise in bile acid chemistry. The company's lead product candidate, obeticholic acid ("OCA"), is a bile acid analog and first-in-class agonist of the farnesoid X receptor ("FXR"). OCA is being developed for a variety of chronic liver diseases including primary biliary cirrhosis ("PBC"), nonalcoholic steatohepatitis, portal hypertension, bile acid diarrhea and primary sclerosing cholangitis ("PSC"). OCA has received orphan drug designation in both the United States and Europe for the treatment of PBC and PSC. Intercept owns worldwide rights to OCA outside of Japan and China, where it has out-licensed the product candidate to Dainippon Sumitomo Pharma. For more information about Intercept, please visit the Company's website at: www.interceptpharma.com.
CONTACT:
Barbara Duncan or Senthil Sundaram
Intercept Pharmaceuticals
1-646-747-1000
Media inquiries: media@interceptpharma.com
Investor inquiries: investors@interceptpharma.com
(click to enlarge)
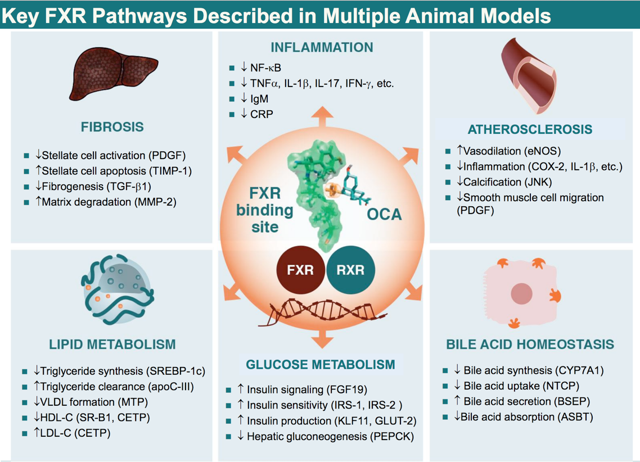
Source: Intercept
It is of great interest (for both investors and patients) that the activation of FXR via OCA amplifies the liver's unique regenerative capability by multiple-folds. The liver is an organ that differs from other organs like the heart, because liver tissues have the unique capability to regenerate themselves. I believe that OCA was intelligently designed for the drug has the power to unlock the human body's untapped regenerative power. Hence, this ingenuity was reflected in the early stoppage on the Flint trial. In addition, it would be reflected in the final outcome data (plus the post-marketing data) for NASH in terms of both efficacy and safety.
NASH is a condition affecting nearly 6 million patients in the US. The medical community does not have an exact understanding pertaining to the cause for NASH per se. Approximately 10% of patients suffering from NASH would eventually develop the potentially lethal disease (liver cirrhosis) - A condition that physicians do not currently have any treatment that is both safe and efficacious.
The ultimate treatment, the liver transplant, is not ideal because not all patients could receive the transplant. This is not to mention there are significant adverse effects from taking post-transplant drugs.
Pertaining to Flint, The trial explores the therapeutic indication of OCA in treating NASH. Flint was halted on January 9, 2014 due to the superb results from the interim data. Accordingly, OCA achieved a highly statistical significant p-value of 0.0024, which beat the required threshold p-value of 0.00305 by far. Currently, OCA is in its phase II study and the final outcome data for Flint would be release around September 2014.
In anticipating the future of OCA, many people believed that OCA would need to demonstrate the ability to reverse liver fibrosis to gain the FDA's approval. Contrarily, OCA would only need to achieve the primary outcomes in Flint to receive approval by the Agency per se.
The primary outcome for NASH, based on liver biopsies studies, include (1) stopping the progression of liver fibrosis (2) improving NAFLD Activity Score ("NAS") by at least two points. The threshold for statistical significance pertaining to the final outcome data was set at the p-value of 0.049. Given the fact that OCA posted superb p-value of 0.0024 for the interim data, I am confident that the hurdle set for OCA in the final outcome data is one that could be easily cleared by the drug. In addition, OCA would surprise both the investing/medical communities in its ability to reverse fibrosis… due to its highly potent anti-inflammatory properties.
(click to enlarge)
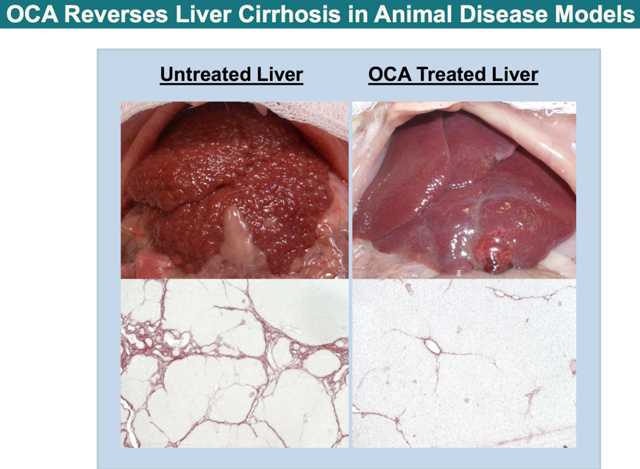
Source: Intercept
While most attention was drawn to NASH due to the interim results in Flint, I believe that another catalyst deserves equal attention. Accordingly, the data for the POISE trial -currently in phase III studying the indication for OCA in treating primary biliary cirrhosis ("PBC") - would be released around June this year.
In December 2013, the last patient followup visit was completed, marking the conclusion of the doubleblind phase of the POISE trial. POISE has been designed to study the safety and efficacy of OCA in PBC patients with an inadequate therapeutic response to ursodiol or who are unable to tolerate ursodiol. In this trial, eligible PBC patients that were currently taking a stable therapeutic dose of ursodiol continued their ursodiol treatment and were randomized into one of three trial arms of approximately 72 patients each, adding either: 10 mg of OCA; 5 mg of OCA increasing over the course of the trial to 10 mg of OCA; or a placebo. The doubleblind phase of the trial was designed to be 12 months in duration, and patients that completed this phase will continue in an open label, longterm safety extension phase for another five years, during which all patients will receive OCA treatment with doses as low as 5 mg and as high as 25 mg a day, as clinically indicated. Of the 217 patients randomized, 19 patients (approximately 9%) discontinued early, including seven patients (approximately 3%) who did so due to pruritus. Of the 198 patients who completed the doubleblind phase, more than 95% continued in the LTSE phase of the trial. We currently expect results from the trial to be available in the second quarter of 2014.PBC is a condition characterized by T-lymphocytes attacking the intralobular ducts of the liver, thus causing signs/symptoms of cholestasis. Untreated cases of PBC eventually leads to liver cirrhosis and liver failure. Symptoms of cholestasis include itching, anemia, vitamin deficiency, malabsorption, metabolic bone diseases, and high cholesterol. Though the exact cause of PBC is unknown, it is believed that PBC might be caused by the body immune system attacking itself. According to Intercept,
While PBC is rare, it is the most common cholestatic liver disease. An estimated 90% of patients are women, with approximately one in 1,000 women over the age of 40 afflicted by the disease. The mean age of diagnosis is about 40 years and the typical initial presentation occurs between the ages of 30 and 65 years. In the United States, the disease is the fifth most common cause of liver transplant and accounts for approximately two percent of deaths attributed to cirrhosis. A majority of PBC patients are asymptomatic at the time of initial diagnosis, but most develop symptoms over time. Fatigue and pruritus, or itching, are by far the most common symptoms in PBC patients. Less common symptoms include dry eyes and mouth, as well as jaundice, which can be seen in more advanced disease. Based on the guidelines of the American Association for the Study of Liver Disease, or AASLD, and EASL, the clinical diagnosis of PBC is established based on the presence of (i) a positive antimitochondrial antibody, or AMA, a marker of this autoimmune disease seen in up to 95% of PBC patients, and (ii) elevated serum levels of ALP, an enzyme that is released by liver cells in response to the bile acid mediated toxicity and that is a key biomarker of the disease pathology. ALP is routinely measured in blood tests and, in the earlier stages of PBC, it is often the only abnormally elevated liver enzyme, rising to between two to ten times higher than normal values. It is closely monitored in PBC patients as an indicator of treatment response and prognosis. Bilirubin is a marker of liver function and is also monitored in PBC to provide an indication of how well the liver is functioning. Liver biopsy can be used to confirm the diagnosis of PBC, but is not required and is becoming less frequently performed.Current treatments for PBC focus on managing symptoms of cholestasis, as well as, barring the destruction of bile ducts inside the liver. In the words of Raoul Poupon, MD, Professor of gastroenterology and hepatology,
The prognosis of patients with PBC has improved greatly during the past two decades because of its diagnosis at earlier stages and the widespread use of ursodeoxycholic acid ("UDCA") treatment. As a result, far fewer patients require liver transplantation, and patients with stages I and II PBC appear to have a normal life expectancyUrsodeoxycholic acid or UDCA is the only approved drug to treat PBC. UDCA works by breaking up more detergent bile acids into pool. By lowering the increase in bile acids, UDCA is able to effectively alleviate the symptoms associated with PBC like itching...
In contrast to UDCA, OCA reduces the elevated bile acid by a different mechanism. In specific, OCA activates FXR with 100x potency compared to natural bile acid, namely CDCA. I believe that OCA is not only able to displace the detergent bile acids pool, but it also suppresses the inflammation that causes the destruction of the bile ducts in the liver per se. Hence, I believe that in treating any medical condition, it is more effective to nib the disease at its bud rather than to primarily manage symptoms alone.
Given that UDCA is already efficacious in treating PBC (especially its symptoms), I ventured into the unknown to predict that the combination treatment using OCA plus UDCA would be far more beneficial than the treatment solely using UDCA. Furthermore, I speculate that OCA would deliver refreshing news pertaining to the data to-be-released in the upcoming months.
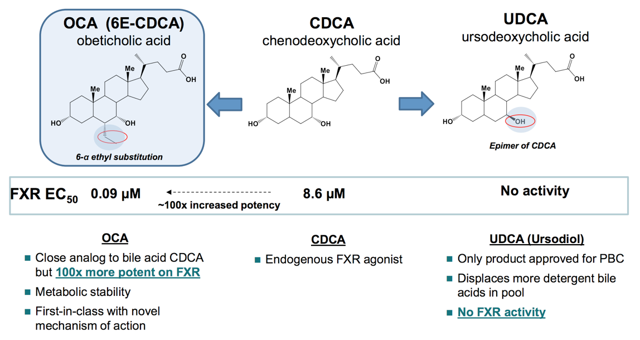
Source: Intercept
Conclusively, there are many short term and longer term catalysts that could levitate or depress the PPS of Intercept Pharmaceuticals. With regards to the closest catalyst, I do not know the specificity that would be discussed at the conference. Nevertheless, I believe that the event would only further educate/inform investors pertaining to the exciting pharmacotherapies at the heart of the company. In my experience of investing in biopharma, I noticed that the PPS tend to move North after such an event due to the accumulating investors' interest in the company.
The bottom line is that if you choose to invest in Intercept, I recommend you to enter your long position before ICPT initiates its next ascent. Unfortunately, I neither guarantee that the PPS would appreciate nor the trials data would turn out stellar. If I could make such promises, investing research would indeed become a perfect science. Investing in biopharmaceuticals entails significant risks versus rewards. It is your responsibility to make that decision based on your level of risk tolerances. Nevertheless, I am here to help you to cut through the maze of biopharmaceuticals investing to the best of my God given abilities. On the final note, if you are an investor in Intercept, you should hold on to your shares for the long term in order to fully profit from this rare opportunity. If I may diverge, I started a group consisted of MD/PhDs to study binary events many years ago. One of the guys in the group bought Jazz Pharmaceuticals at an average PPS below $1. He sold it prematurely for it increased several folds in PPS. Despite such drastic appreciation in shares price, Jazz continued to increase the PPS of $143 as of this writing. My friend had kicked himself in the foot since.
Additional disclosures: I would like to thank a special friend, who is an EM/IM physician. He helped me to understand NASH and to improve my data analysis. This selfless genius had been there for me when I had nothing going on for me in my life. Yet I have not been able to repay him for his help. Without his guidance, as well as, the guidance from my previous colleagues, mentors, bosses, friends, family, and TP, I would not be here today.
No comments:
Post a Comment